Real-world data and real-world evidence are used to monitor the post-market safety and adverse events of drugs. The monitoring of this data assists in making regulatory decisions. The healthcare community is using RWE and RWD to support coverage decisions and to develop guidelines and decision support tools for use in clinical practice.
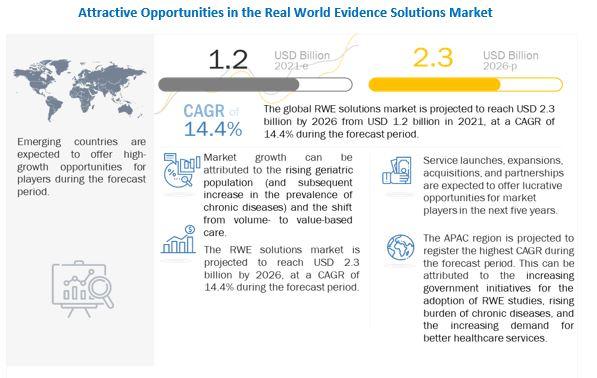
The rising geriatric population (and the subsequent increase in the prevalence of chronic diseases) is a key factor driving the growth of this market. The shift from volume- to value-based care, delays in drug development (and the subsequent increase in development costs).
𝐆𝐞𝐭 𝐌𝐨𝐫𝐞 𝐈𝐧𝐬𝐢𝐠𝐡𝐭𝐬, 𝐆𝐫𝐚𝐛 𝐏𝐃𝐅 @ https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=76173991
The emergence of this pandemic has posed severe financial constraints on pharma-biopharma companies in several countries. In this regard, RWE solutions have proven to be very helpful, as they allow industrial and academic researchers to monitor patients using digitally connected platforms while helping to organize and evaluate clinical data for regulatory submissions.
The uncertainty brought on by the COVID-19 pandemic has dramatically shifted how and when patients decide to seek medical care. In addition, shifts in healthcare coverage and provision during the pandemic have changed the discovery and reporting of certain outcomes in data and the treated population.
Even though emerging technologies, such as telemedicine, have existed for decades, most of healthcare systems rely heavily on in-person interactions between patients and clinicians. Nevertheless, the current requirement for social distancing measures is swiftly pushing the primary care provision toward remote care.
Telemedicine and virtual care may also prompt a greater adoption of technologies such as wearables and digital therapeutics, thus accelerating digitalization in the healthcare space and boosting the importance of RWE and AI.
Regulators use real world evidence market to monitor the safety of marketed products through traditional pharmacovigilance tools (for instance, Periodic Benefit-Risk Evaluation Report, Periodic Safety Update Report, and Vaccine Adverse Event Reporting System) as well as newer digital aids such as the FDA Sentinel Initiative, a post-market active safety surveillance system.
