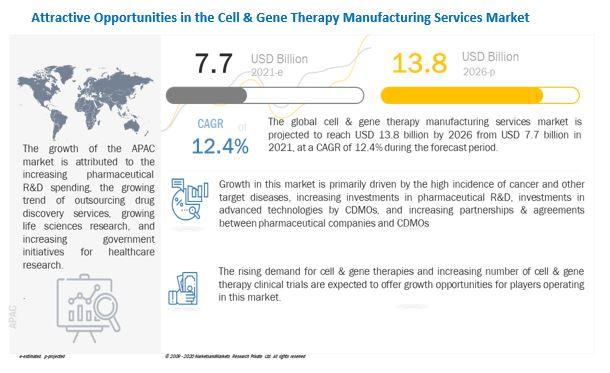
Growth in cell & gene therapy manufacturing services market is primarily driven by the high incidence of cancer and other target diseases, increasing investments in pharmaceutical R&D, investments in advanced technologies by CDMOs, and increasing partnerships & agreements between pharmaceutical companies and CDMOs.
The high operational costs associated with cell & gene therapy manufacturing are expected to restrain the growth of cell & gene therapy manufacturing services market to a certain extent.
Most pharmaceutical companies continue to invest heavily in the development of novel drugs and devices. The pharmaceutical industry, in particular, is R&D-intensive. Pharmaceutical companies invest in R&D to deliver high-quality and innovative products to the market.
𝐆𝐞𝐭 𝐌𝐨𝐫𝐞 𝐈𝐧𝐬𝐢𝐠𝐡𝐭𝐬, 𝐆𝐫𝐚𝐛 𝐏𝐃𝐅 @ https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=180609441
The increase in pharmaceutical R&D has resulted in a sharp increase in the number of cell & gene therapy candidates under development. This has made it necessary to outsource manufacturing services to develop cost-effective and efficient cell & gene therapies.
Currently, there are 1,200 cell & gene therapies in trials worldwide. There are more than 700 investigational cell & gene therapies in clinical development in the US alone. However, manufacturing facilities have not kept up. It has been estimated that hundreds of facilities will be needed to manufacture the treatments that are now in clinical trials.
The cost of manufacturing for gene therapy can be between USD 500,000 and USD 1 million, excluding the costs for R&D, the costs to run crucial clinical trials, or the costs to build the commercial infrastructure necessary to provide access to patients.
Clinical trials are the backbone of medical research and help pharmaceutical and biopharmaceutical companies develop and commercialize new cell & gene therapies. In the past few years, the demand for clinical trials has risen worldwide as a result of the growing demand for novel medicines to fulfill unmet medical needs. According to a 2020 PhRMA report on the cell & gene therapy pipeline in 2018, there were 289 cell & gene therapies in clinical development by biopharmaceutical companies.