Real-world data and real-world evidence are used to monitor the post-market safety and adverse events of drugs. The monitoring of this data assists in making regulatory decisions. The healthcare community is using RWE and RWD to support coverage decisions and to develop guidelines and decision support tools for use in clinical practice.
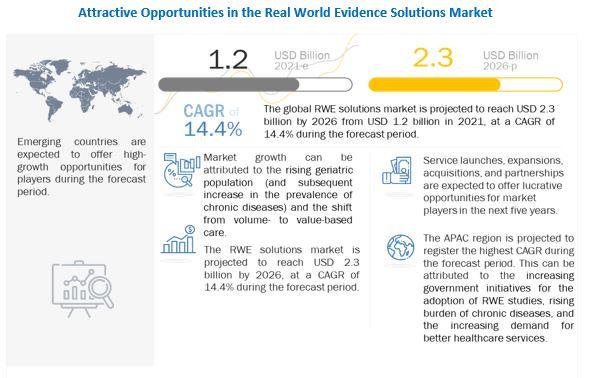
The rising geriatric population (and the subsequent increase in the prevalence of chronic diseases) is a key factor driving the growth of this market. The shift from volume- to value-based care, delays in drug development (and the subsequent increase in development costs), growth in R&D spending, and support from regulatory bodies for the use of RWE solutions are some of the other major factors that are driving the growth of this market.
The emergence of this pandemic has posed severe financial constraints on pharma-biopharma companies in several countries. In this regard, RWE solutions have proven to be very helpful, as they allow industrial and academic researchers to monitor patients using digitally connected platforms while helping to organize and evaluate clinical data for regulatory submissions.
Download PDF Brochure @ https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=76173991
The uncertainty brought on by the COVID-19 pandemic has dramatically shifted how and when patients decide to seek medical care. In addition, shifts in healthcare coverage and provision during the pandemic have changed the discovery and reporting of certain outcomes in data and the treated population.
RWE is set to become the most influential emerging technology to help in the fight against the COVID-19 outbreak, according to the latest poll on GlobalData’s Pharmaceutical Technology website. In this poll, which was completed by 935 of its readers in April 2021, more than one-third of the respondents indicated that RWE would have the greatest impact on the management of COVID-19.
Even though emerging technologies, such as telemedicine, have existed for decades, most of healthcare systems rely heavily on in-person interactions between patients and clinicians. Nevertheless, the current requirement for social distancing measures is swiftly pushing the primary care provision toward remote care. Telemedicine and virtual care may also prompt a greater adoption of technologies such as wearables and digital therapeutics, thus accelerating digitalization in the healthcare space and boosting the importance of RWE and AI.
The utilization of RWE in infectious disease control is not a new concept. During the Ebola outbreak in 2014, forecasters successfully used Global Epidemic and Mobility (GLEaM) simulations that combined real-world data on populations and their mobility with rigorous stochastic models of disease transmission to predict the global spread of the disease.
Mobile contact-tracing apps have played an important role in easing the spread of COVID-19 in China. Government-backed apps analyzed personal data to group individuals into color-coded categories corresponding to their health status and level of risk to contract COVID-19. Following success in China, an increasing number of countries have started to look for ways to implement similar measures. In countries with strict data privacy laws, the implications of contact-tracing apps on individual privacy are considered a major associated concern.
Key Market Players
Prominent players in the real world evidence solutions market include IQVIA (US), IBM Corporation (US), ICON plc. (Ireland), PAREXEL International Corporation (US), PPD, LLC (US), Optum, Inc. (US), Cognizant Technology Solutions Corporation (US), Oracle Corporation (US), SAS Institute Inc. (US), Syneos Health, Inc. (US), Anthem, Inc. (US), Clinigen Group plc. (UK), Medpace Holdings Inc. (US) and Flatiron Health, Inc. (US).
