The emergence of this pandemic has posed severe financial constraints on pharma-biopharma companies in several countries. In this regard, RWE solutions have proven to be very helpful, as they allow industrial and academic researchers to monitor patients using digitally connected platforms while helping to organize and evaluate clinical data for regulatory submissions.
RWE is set to become the most influential emerging technology to help in the fight against the COVID-19 outbreak, according to the latest poll on GlobalData’s Pharmaceutical Technology website. In this poll, which was completed by 935 of its readers in April 2021, more than one-third of the respondents indicated that RWE would have the greatest impact on the management of COVID-19.
For More Info, Download PDF Brochure @ https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=76173991
[215 Pages Report] Real-world data and real-world evidence are used to monitor the post-market safety and adverse events of drugs. The monitoring of this data assists in making regulatory decisions. The healthcare community is using RWE and RWD to support coverage decisions and to develop guidelines and decision support tools for use in clinical practice.
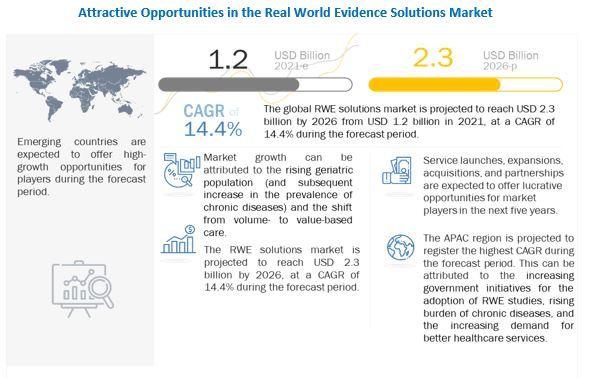
The global RWE World Evidence Solutions Market is projected to reach USD 2.3 billion by 2026 from USD 1.2 billion in 2021, at a CAGR of 14.4% during the forecast period. The rising geriatric population (and the subsequent increase in the prevalence of chronic diseases) is a key factor driving the growth of this market.
The shift from volume- to value-based care, delays in drug development (and the subsequent increase in development costs), growth in R&D spending, and support from regulatory bodies for the use of RWE solutions are some of the other major factors that are driving the growth of this market.
Accelerated digitalization in the healthcare space has revealed gaps in infrastructure, workforce, and digital education that ultimately need to be bridged. Without intelligent analytics, RWE alone will not be able to produce meaningful and actionable results. Previously, the healthcare industry did not have the ability to gather RWE at the speed and scale needed to address urgent public health crises.
With almost 80% of clinical trials failing to meet their initial enrollment projections, delays in patient recruitment can cripple a trial and R&D program. Healthcare providers are increasingly investing in health information technologies, which is permitting them to better track and connect with patients.
