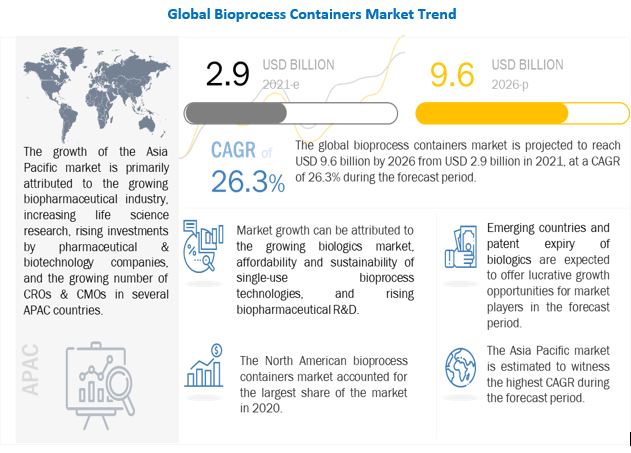
Future Growth in the bioprocess containers market is mainly driven by factors such as the growing biologics market, affordability and sustainability of single-use bioprocess technologies, and rising biopharmaceutical R&D. Emerging countries and the patent expiry of biologics are expected to offer growth opportunities for players operating in the bioprocess containers market during the forecast period.
COVID-19 is an infectious disease caused by the most recently discovered novel coronavirus. Largely unknown before the outbreak began in Wuhan (China) in December 2019, COVID-19 has since moved from being a regional crisis to a global pandemic.
Therefore, the growing adoption of single-use technologies for COVID-19 pandemic-related research, coupled with increasing vaccine development by key players, is expected to have a positive impact on the bioprocess containers market.
Download PDF Brochure @ https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=107645832
In recent years, more biologics having higher potency that require smaller production volumes, advancements in biosimilars targeting smaller markets, and ongoing improvements in production yields and efficiencies that create production operations at much smaller scales have increased the use of single-use technologies such as single-use bioprocess containers/bags as they are best suited for small volume productions.
Additionally, the Centre for Biologics Evaluation and Research’s (CBER) (2021-25) strategic plan involves various schemes for boosting the growth of the biologics market. With increasing growth in the biologics market, the number of bioprocess containers needed for R&D and biologics manufacturing is also expected to increase in the coming years.
According to a survey conducted by BioPlan Associates in 2018, 73.3% of respondents agreed that leachables and extractables are major concerns that may limit the use of bioprocessing in the near future. As single-use assembly products are made of processed plastic materials, they often face the problem of contamination from the container due to leachables.
Furthermore, a majority of biopharmaceutical processes frequently require high temperatures during their operation, which results in the generation of unnecessary and unknown chemicals that may contaminate the end product. Thus, concerns regarding extractables and leachables arising from the containers may hinder their adoption to a certain extent in the coming years.
The bioprocess containers market is dominated by a few globally established players such as Sartorius Stedim Biotech (France), Thermo Fisher Scientific (US), Danaher Corporation (US), and Merck Millipore (Germany).
